Nuvaxovid
Information about NUVAXOVID SARS-CoV-2 recombinant spike protein authorized by Health Canada as a Vaccine for COVID-19. First Approval of the Protein-Based Adjuvanted Nuvaxovid NVX-CoV2373 Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
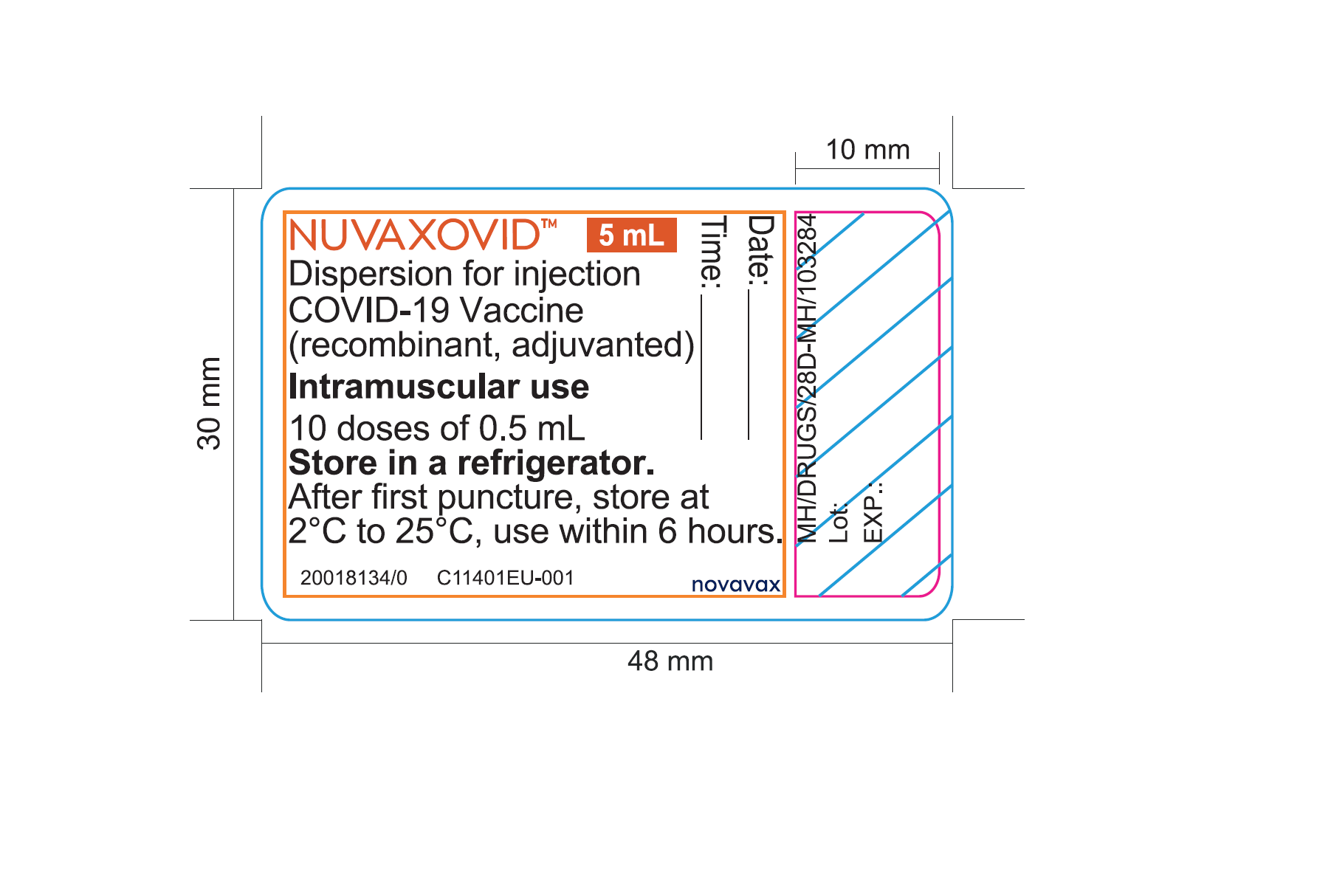
. Nuvaxovid has manufacturing sites in the Czech Republic Australia Canada Japan and South Koreas SK. This type of vaccine contains part of the coronavirus spike protein. The flu vaccine and the hepatitis B vaccine which.
The use of this vaccine should be in accordance with. The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
The addition of the saponin-based. Nuvaxovid contains a version of a protein found on the. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19.
The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its. Novavaxin Nuvaxovid-koronarokote antaa suojaa SARS-CoV-2 viruksen aiheuttamaa infektiota ja COVID. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
19 2022 Novavax signed agreements with. Most important facts about the new vaccine Nuvaxovid. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes.
Nuvaxovid is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 years of age and older. WHO lists 10th COVID-19 vaccine for emergency use. Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older.
After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria. Cambridge Mass and Osaka Japan April 19 2022 Takeda today announced that it has received manufacturing and marketing approval from the Japan Ministry of Health. Your immune system cells recognise the spike protein as a threat and begin building an immune response.
A full list of ingredients for the qualitative and quantitative. Unlike mRNA vaccines such as Pfizer and Moderna the Novavax vaccine uses a longer-standing protein-based technology. The MHRA can confirm that Nuvaxovid does not contain any components of animal origin.

Distribution Of Nuvaxovid With English Only Vial And Carton Labels Canada Ca
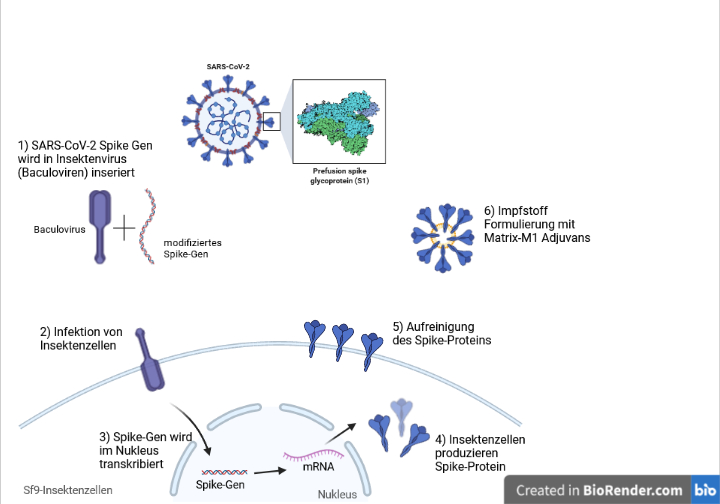
Nuvaxovid The New Subunit Sars Cov 2 Vaccine Mci Innsbruck

Is The Novavax Covid Vaccine Worth The Hype Medpage Today
Switzerland Approves Novavax S Covid Vaccine For 12 18 Year Olds Swi Swissinfo Ch

Ema Plans Anaphylaxis Label On Novavax Covid 19 Vaccine

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care

Novavax S Vaccine Nuvaxovid Vaktsineeri Ee

Novavax Requests Expanded Emergency Use Listing With Who For Nuvaxovid Covid 19 Vaccine For Adolescents Aged 12 Through 17 Eatg
Fda Authorizes Novavax Covid 19 Vaccine For Emergency Use In Us Abc News
After A Decent First Quarter Novavax S Covid Shot Is Struggling

Infomesen Long Covid 19 Nuvaxovid Novavax Vaksin Australian Government Department Of Health And Aged Care
Faq What You Need To Know About Novavax S Non Mrna Covid 19 Vaccine Nuvaxovid Cna

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl

Nvx Cov2373 Recombinant Adjuvanted Covid 19 Vaccine

Novavax Covid 19 Vaccine Nuvaxovid Data On Side Effects

Fda Advisers Overwhelmingly Endorse Novavax Covid 19 Vaccine Ars Technica

Coronavirus Q A On The Nuvaxovid Covid 19 Vaccine Cyprus Mail

Novavax S Covid 19 Vaccine Candidate Receives Ec Approval For Use As A Booster Pmlive

